Materials
- Photovoltaic cell (3V min) or 9-volt battery
- (2) Pieces of aluminum foil 6 cm x 10 cm
- Salt
- (2) Electrical wires with 2 cm uninsulated on each end
- (2) Paper clips
- Small bowl (glass, ceramic or plastic)
- Water
- Spoon

Background
Electrolysis is a technique used by scientists to separate a compound or molecule into its component parts. By adding electricity to water and providing a path for the different particles to follow, it can be separated into hydrogen and oxygen.
Procedure
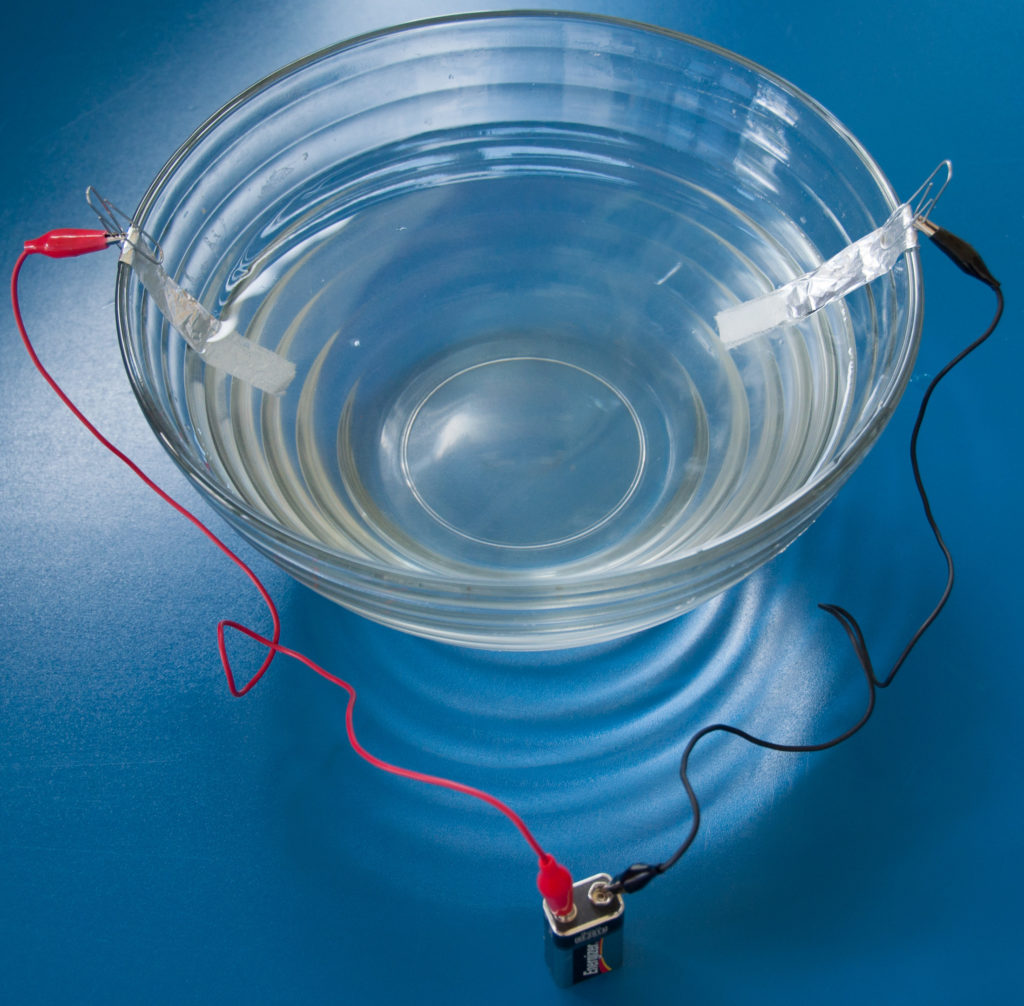
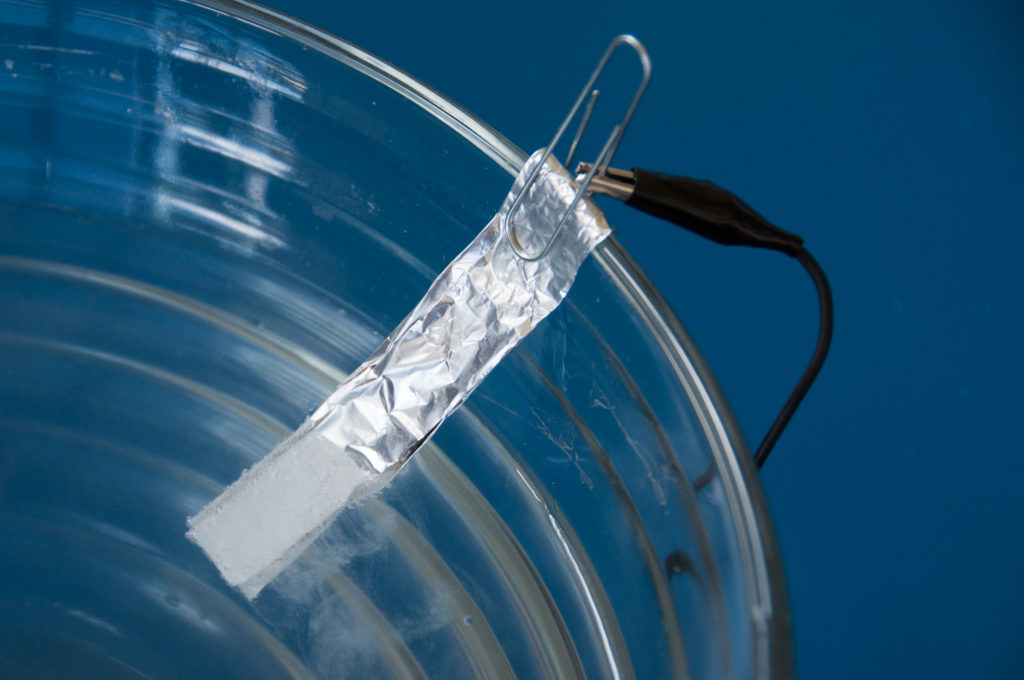
- Accordion-fold each piece of aluminum foil down the long way so that you have two pieces approximately 1 cm x 6 cm. These are going to be your electrodes.
- Press each electrode flat.
- Bend the top 1 cm of each electrode over to act as a hanger. They will be hung on the inside of your bowl.
- Attach one end of each wire to the hanger of your electrode with a paper clip.
- Dissolve salt into water at the ratio of one teaspoon salt for each 50 ml of water. Stir to dissolve the salt.
- Hang the electrodes on the inside of the bowl so that they hang down into the water. They should hang a couple inches apart; do not let them touch during the experiment. Add more salt water if necessary.
- Attach the other end of each wire to your photovoltaic panel or a battery. Make a note which electrode is attached to the positive and which is attached to the negative.
- If using photovoltaics, take your electrolysis device outside into the sun.
- Observe the electrolysis procedure and what is occurring at each electrode.