The hydrogen and fuel cell laboratories at the FSEC Energy Research Center at the University of Central Florida are one of the nation’s highest caliber research labs of its kind. The Center conducts hydrogen research on production, storage, utilization and sensors, and fuel cell research on membranes, fabrication, mass transport, surface properties and electrochemical diagnostics.
Fuel Cell Laboratory
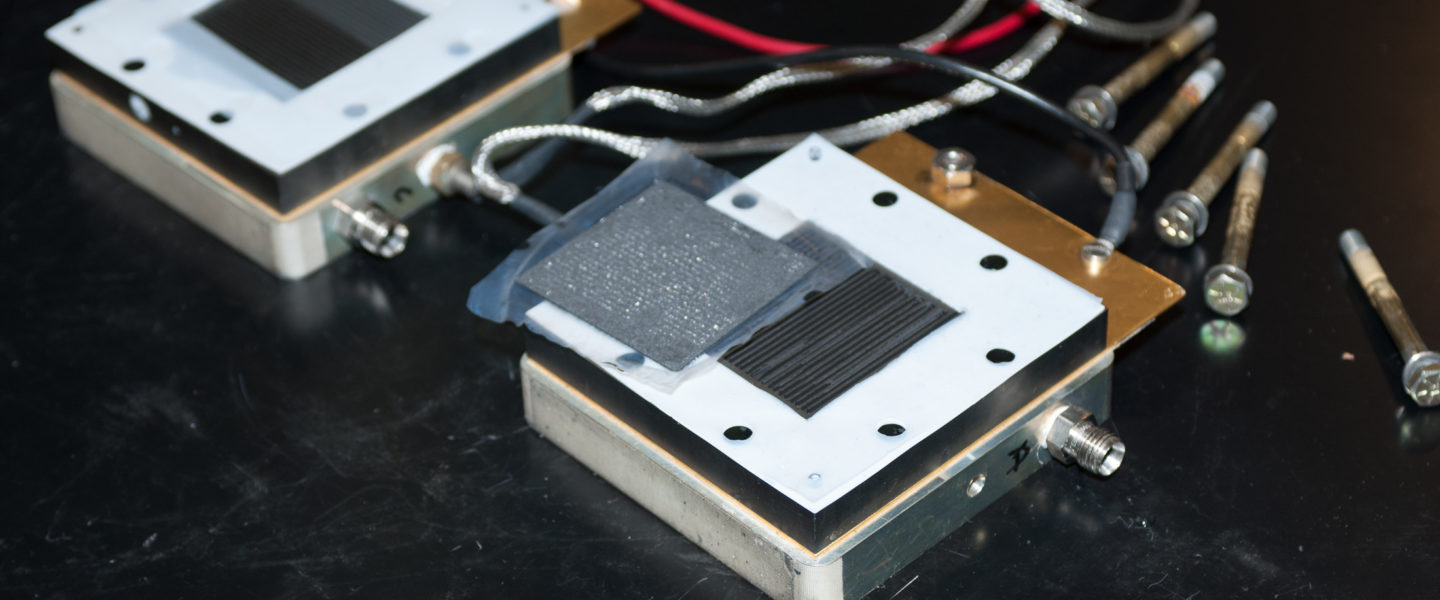
The focus of the Fuel Cell Laboratory is to develop techniques that reduce membrane degradation in a fuel cell application. This lab has the ability to fabricate nearly every component of a fuel cell—from the membrane, to the catalyst ink, to the final membrane electrode assembly (MEA). Researchers can evaluate performance or durability of an assembled fuel cell using temperature, gas concentrations and relative humidity on single-cell test stands, or a one-of-a-kind multi-station durability test station.
Membrane Electrode Assembly Fabrication
Catalyzed membranes—both supported and unsupported for fuel cell applications—are fabricated in the Membrane Electrode Assembly (MEA) Fabrication Facility. Lab equipment includes: convection ovens, vacuum ovens, high temperature tube furnaces, and an ultrasonic blender to synthesize the catalytic materials. The fabrication facility has:
- Special apparatus for casting polymer membranes and synthesizing blends and composites
- Spray stations for spraying the catalyst, either manually or on an XY table
- Screen-printing machine to make membrane electrode assemblies using the decal method.
In-Situ Electrochemical Diagnostics
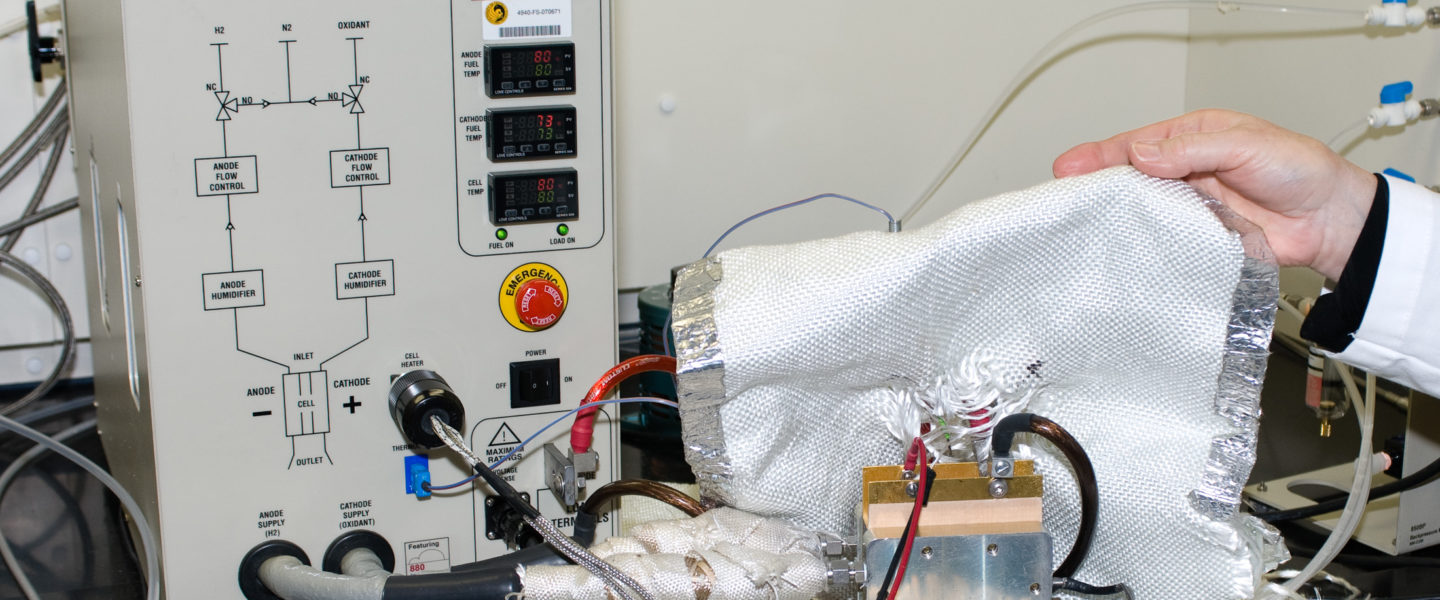 In-situ electrochemical diagnostics is performed with a fuel cell test stand, a load box and a frequency response analyzer that comprise a complete fuel cell test station. The tests can be done using 5cm2, and 25cm2 single cell hardware. Current-voltage performance data quantify the decrease in cell voltage with increasing current density. Catalytic activity is measured in mA/mg-Pt, at a cell voltage selected to minimize effects of reactant gas crossover and mass-transport limitation. Current-interrupt measurements include membrane ionic resistance, and contact resistance within, and between, MEA layers.
In-situ electrochemical diagnostics is performed with a fuel cell test stand, a load box and a frequency response analyzer that comprise a complete fuel cell test station. The tests can be done using 5cm2, and 25cm2 single cell hardware. Current-voltage performance data quantify the decrease in cell voltage with increasing current density. Catalytic activity is measured in mA/mg-Pt, at a cell voltage selected to minimize effects of reactant gas crossover and mass-transport limitation. Current-interrupt measurements include membrane ionic resistance, and contact resistance within, and between, MEA layers.
Membrane reactant crossover rate and ionic resistance are important measures of membrane quality. Hydrogen supplied to the MEA reference electrode permeates the membrane and reaches the opposite electrode at a rate that is measured by the limiting hydrogen oxidation current. Typical rates of reactant crossover are less than 4mA/cm2. The current-interrupt technique and AC impedance spectroscopy measure membrane ionic resistance.
The extent and electrochemical activity of the interfacial area where polymer electrolyte, catalyst, and reactants meet within the electrode, and the electrode permeability to reactant gas, are important measures of electrode quality. Cyclic voltammetry measures the electrochemically-active area, and the electrochemical stability. Hydrogen desorption, or carbon monoxide titration, yield the electrochemically-active interfacial area in m2/g-Pt, which quantifies catalyst utilization. The double-layer charging current yields double-layer capacitance, and can be analyzed to reveal available interfacial area. Current-voltage limiting current data—acquired using oxygen, diluted to various extents with nitrogen or with helium—characterize electrode reactant gas permeability and identify diffusion processes, such as Knudsen diffusion within pores, or multi-component bulk diffusion.
Areas of Expertise
- Membrane manufacturing
- Membrane testing
- Conductivity
- Ex-situ durability
- Stress-strain
- Electrode ink manufacturing
- Electrode ink application methods
- Direct spray
- Decal transfer
- Fuel cell testing capabilities
- Performance of single cells (up to 50A)
- Durability (cycling and OCV hold)
- Analytical techniques
- LSV
- CV
- IR-camera for LCO (give example images)
- Flow battery testing
- Vanadium flow battery results
Hydrogen Dry Laboratory (Analytical Instrumentation)
 The Hydrogen Dry Laboratory houses many of the analytical tools that are required for successful completion of any research project. These tools enable researchers to evaluate the physical properties of the research materials, from physical morphology to chemical stability. Other projects supported in this lab include: hydrogen sorption in metal hydrides, fluoride analysis from fuel cell effluent water, oxygen reduction reaction rates of novel fuel cell catalysts, and thermal stability of electrolyte membranes. Areas of experise include:
The Hydrogen Dry Laboratory houses many of the analytical tools that are required for successful completion of any research project. These tools enable researchers to evaluate the physical properties of the research materials, from physical morphology to chemical stability. Other projects supported in this lab include: hydrogen sorption in metal hydrides, fluoride analysis from fuel cell effluent water, oxygen reduction reaction rates of novel fuel cell catalysts, and thermal stability of electrolyte membranes. Areas of experise include:
- TM 3000 SEM
- Ion chromatography systems for anion and cation analysis
- TGA-MS
- Polymer decomposition and analysis
- DSC
- Thermal transitions of polymers
- Gas chromatography
- Rotating disc electrode
- Ideal for characterizing catalyst ORR activity
- FTIR
- UV-Vis
- Used for determining state of charge of vanadium flow battery system
Chemical Synthesis Laboratory

In the Chemical Synthesis Laboratory, also known as the Hydrogen Wet Lab, UCF researchers have:
- Developed hydrogen chemochromic tape
- Synthesized novel SPEEK membranes for fuel cell applications
- Developed an approach for conversion of biomass to diesel through Fischer-Tropsch gasification.
Cryogenic Hydrogen Laboratory
 In the Cryogenic Hydrogen Lab, UCF researchers have developed a lab-scale hydrogen liquefaction and zero boil-off storage system, and a highly-efficient GM and Stirling-type pulse tube refrigerator for space application. UCF researchers have also:
In the Cryogenic Hydrogen Lab, UCF researchers have developed a lab-scale hydrogen liquefaction and zero boil-off storage system, and a highly-efficient GM and Stirling-type pulse tube refrigerator for space application. UCF researchers have also:
- Developed an approach to liquefy hydrogen efficiently
- Developed a cryocooler-cooled hydrogen liquefier and cryogen management system.
Researchers have extensively explored liquid hydrogen-related systems engineering in all aspects of thermal design, structural analysis, fabrications, and testings, including:
-
-
- Cryogenic propellant densification with integrated cryocooler at NASA KSC (NASA GRC)
- Cryocooler integrated zero-boil-off densified liquid hydrogen storage tank (NASA GRC)
- Liquid hydrogen calorimeter test and zero-boil-off test (NASA KSC)
- Thermal analysis of NASA KSC GODU liquid hydrogen with an integrated refrigerator.
-
