 Methanol is one of the chemicals suggested to be a promising low-cost hydrogen carrier for its high energy density, ease of transportation, and lower risk compared to ammonia. To generate hydrogen at point of use, methanol needs to be converted into H2, which can be done thermally via steam methane reforming (SMR) or electrochemically via electrolysis. UCF researchers collaborate with industry partners who produce low-carbon methanol from distributed sources and investigate how impurities in crude methanol impact the performance of methanol electrolysis.
Methanol is one of the chemicals suggested to be a promising low-cost hydrogen carrier for its high energy density, ease of transportation, and lower risk compared to ammonia. To generate hydrogen at point of use, methanol needs to be converted into H2, which can be done thermally via steam methane reforming (SMR) or electrochemically via electrolysis. UCF researchers collaborate with industry partners who produce low-carbon methanol from distributed sources and investigate how impurities in crude methanol impact the performance of methanol electrolysis.
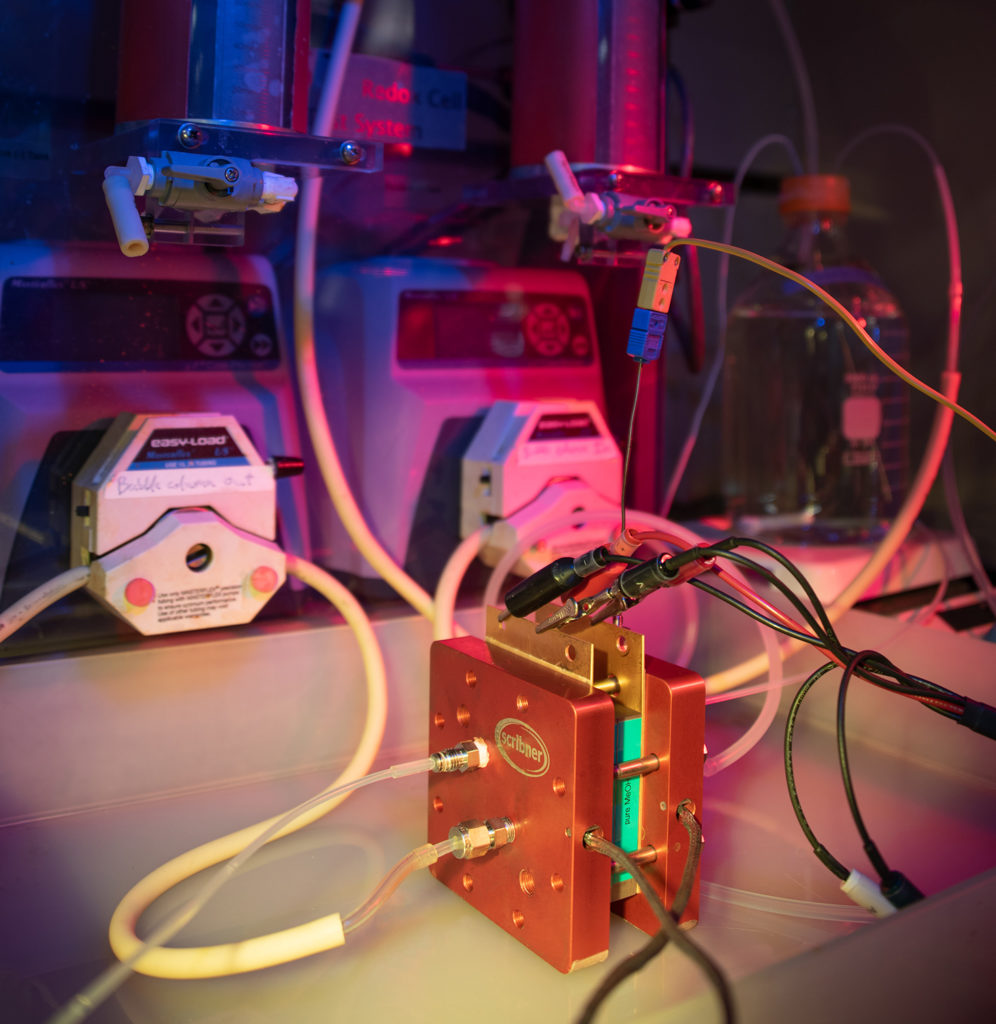
With state-of-the-art commercial catalysts, methanol electrolysis requires less electricity than water electrolysis due to its lower onset potential. This allows methanol electrolysis to be deployed at places that have higher electricity costs. At FSEC, we evaluate the economy and the carbon intensity of the integrated process from methanol production to methanol electrolysis. One important question to answer is whether there is an economic or environmental incentive for crude methanol purification for such pathway. To answer this question, we use a bench-scale membrane electrode assembly (MEA) to evaluate the energy penalty that results from the impurities in crude methanol. This experimental data can then be further used in a sensitivity analysis to build models that can help us determine the most economic pathway using methanol produced from decentralized producers as a hydrogen carrier.
Year of Research: 2023
Sponsored by: M2X Energy Inc. & Florida High Tech Corridor